As a result 20 moles of helium He represent 20 mol 6022 1023 12 1024 atoms. 12x1024 molecules glucose x 1 mole 6022x1023 molecules 20 moles glucose 4.
How many sodium ions are in 30 moles of NaCl.

. How many sodium atoms are in 30 moles of NaCl. Jun 19 2010 1 mole He is 602 x 1023 atoms He. 5 moles to atom 3.
Type in your own numbers in the form to convert the units. As a result 20 moles of helium He represent 20 mol 6022 1023 12 1024 atoms. How many moles of water does 602 x 1023 molecules represent.
How many total atoms are in 80 moleS of H2O. Submit order and get a quick answer at the best price for any assignment or question with DETAILED EXPLANATIONS. What is the mass of 200 mol of nickel.
How many atoms are in 200 moles of H2O. How many sodium ions are in 30 moles of NaCl. How many atoms are present in 1 mole of chromium.
We use the following formula to calculate the number of atoms. What does this mean. The mass of NA nickel atoms is 587gmol1.
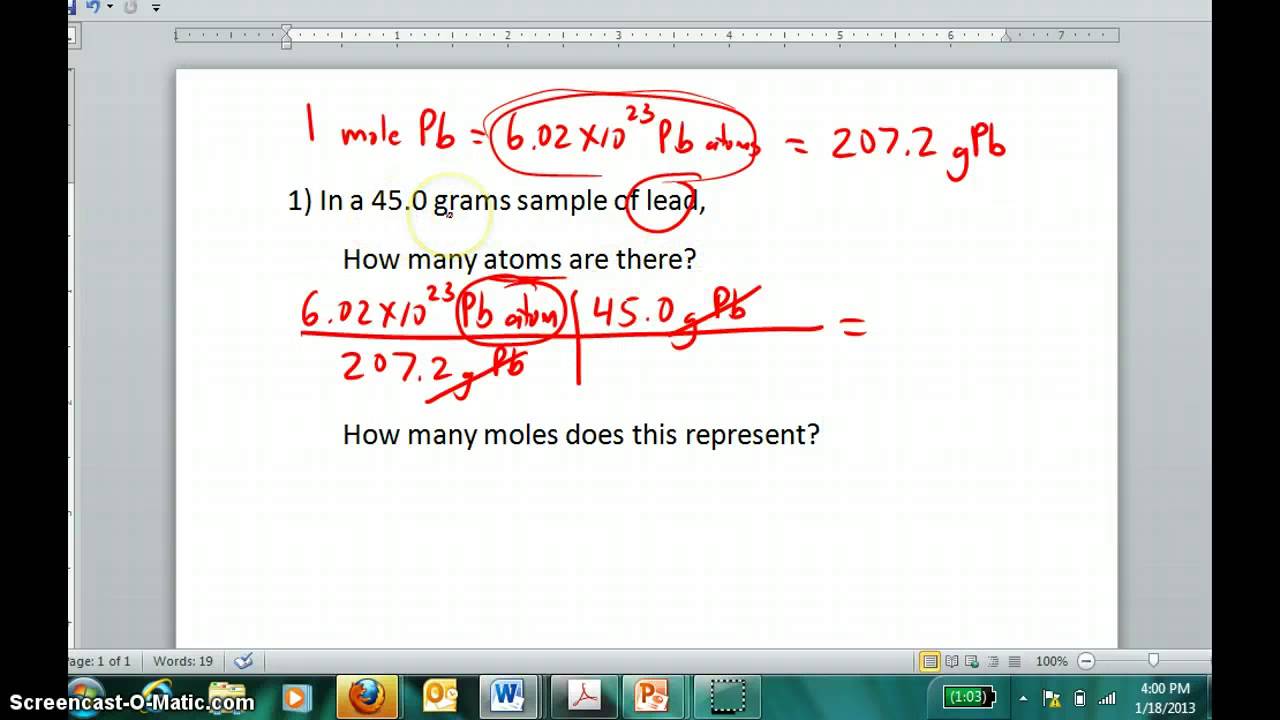
In one mole of any element there is Avogadros number of atoms which is 602 x 1023. What ions are formed when CaCl2 dissolves in water. 0259 417 297 48 63 12 102 2678 95 102 g g g g g g g g.
How many total atoms are in 80 moleS of H2O. 6 How many sodium ions are in 30 moles of NaCl. 7 How many molecules are in 025 moles of CH 4.
Quick conversion chart of moles to atom. How many sodium ions are in 30 moles of NaCl. 3 moles to atom 180664245E24 atom.
How many atoms does 20 moles of He represent. How many total atoms are in 10 moles of H2O. Use this page to learn how to convert between moles and atoms.
The molar mass of elemental chromium is 5200gmol1. Up to 24 cash back How many atoms does 20 moles of He represent. Convert 301 x 1023 molecules of C2H6 to moles.
According to Avogadros number 1 mol of any substance contains 6022 1023 particles. 4 moles to atom 24088566E24 atom. How many atoms does 2 moles of he represent.
How many sodium ions are in 30 moles of NaCl. How many atoms does 20 moles of He represent. What is the total number of atoms contained in 200 moles of h2o.
As a result 20 moles of helium He represent 20 mol 6022 1023 12 1024 atoms. 4 How many moles of CaCl 2 does 241 x 10 24 formula units represent 5 How many atoms does 20 moles of He represent. Answer in Chemistry for Meagan 50317 Answer to Question 50317 in Chemistry for Meagan Answers Chemistry Other Question 50317 How many moles of CaCl2 does 241 x 1024 formula units represent.
Therefore 2 moles He must be twice that. How many molecules are in 025 moles of CH4. How do you find moles from molarity.
N mol Nnumber of ions N A 1 molecule of NaCl contains 1 sodium ion Na thats why if we have 30 moles of NaCl we have 30 moles of Na. N mol Nnumber of atoms N A NHe nmol N A NHe 20 moles 6021023 12041023 atoms 2. As a result 20 moles of helium He represent 20 mol 6022 1023 12 1024 atoms.
Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. How many moles of CaCl2 does 241 x 1024formula units represent. 8 How many total atoms are in 10 moles of H 2 O.
Convert 301 x 1023 molecules of You May Also Find These Documents Helpful. Experts answer Download Answer Need a fast experts response. How many atoms does 20 moles of He represent.
Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. ℹ HELP. 2 moles to atom 12044283E24 atom.
1 moles to atom 60221415E23 atom. Answers in random order 0250 0500 0911 146 175 200 256 444 moles moles moles moles moles moles moles moles. Experts answer In 1 mole of substance equal602210 23 moleculestherefore N A 602210 23 n025 mol NN A n N602210 23 025 1501023 molecules Need a fast experts response.
Convert 301 x 1023 molecules of C2H6 to moles. How many atoms does 20 moles represent. There are 1806 x 1024 atoms in 3 moles of chromium.
241x1024 units CaCl2 x 1 mole 6022x1023 units 400 moles CaC2 Changing Number of Particles to Mass Round answers correctly and show proper units. With our Periodic Table beside you if you are doing your chemistry homework we find that the molar mass ie. How many moles of water does 602 x 1023 molecules represent.
Are in 10 mole of H2O. How many moles of water does 602 x 1023 molecules represent. Submit order and get a quick answer at the best price.
What is the mass of 075 moles of CuSO4. According to Avogadros number 1 mol of any substance contains 6022 10 23 particles. How many molecules are in 025 moles of CH4.
As a result 20 moles of helium He represent 20 mol 6022 10 23 12 10 24 atoms. How many molecules are in 025 moles of CH4. How many atoms does 20 moles of He represent.
DrBob222 Jun 19 2010 YEET USE CODE LAZAR IN THE FORTNITE ITEM SHOP YEET Jun 26 2020 Respond to this Question Similar Questions chemistry.

Pin By Lynn Cutler On Chemistry Water Molecule Chemistry Molecules

What Is The Total Number Of Atoms Contained In 2 00 Moles Of Nickel Lisbdnet Com
0 Comments